Quality Control of Cosmetics
For beauty that lasts:
Purity and safety in every drop -trust in tested cosmetics!

Effectiveness of Preservatives
We evaluate the effectiveness of preservatives to ensure your cosmetic products are protected from microbial contamination.
Using ISO 11930:2019 guidelines, we provide reliable data on the antimicrobial performance, safeguarding both product integrity and consumer safety.
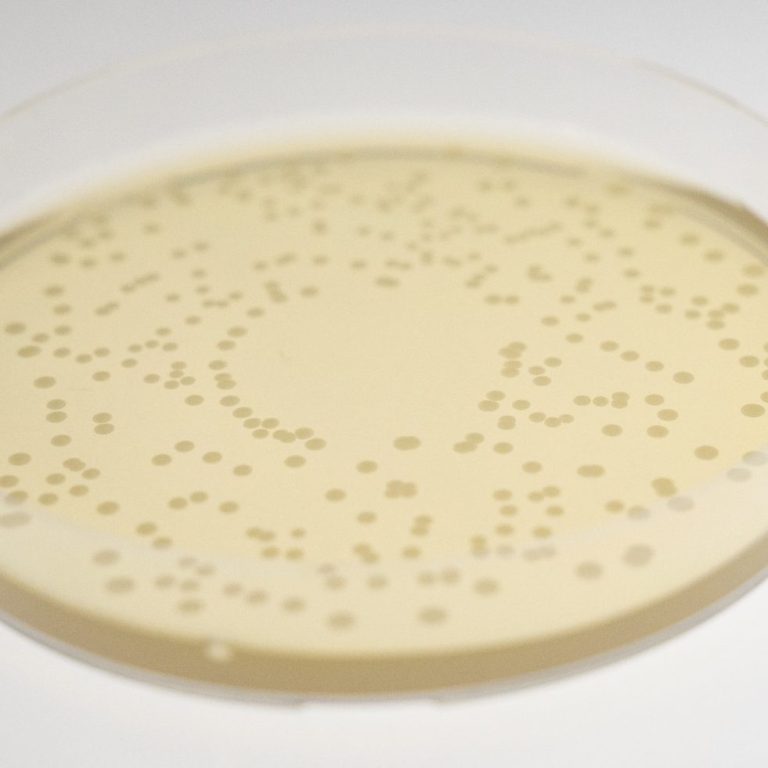
Microbiological Purity Testing
Our laboratory conducts in-depth testing to verify the purity of your products. This includes:
- Enumeration of yeasts and molds (ISO 16212:2008)
- Detection of specified and non-specified microorganisms (ISO 18415:2007)
- Specific tests, such as Candida albicans detection (ISO 18416:2007).

Pyrogen and Endotoxin Testing
We perform pyrogen testing, including bacterial endotoxin testing (LAL test), to ensure products are free of harmful contaminants. This step is crucial for verifying the safety of your formulations.
Wir benötigen Ihre Zustimmung zum Laden der Übersetzungen
Wir nutzen einen Drittanbieter-Service, um den Inhalt der Website zu übersetzen, der möglicherweise Daten über Ihre Aktivitäten sammelt. Bitte überprüfen Sie die Details in der Datenschutzerklärung und akzeptieren Sie den Dienst, um die Übersetzungen zu sehen.

